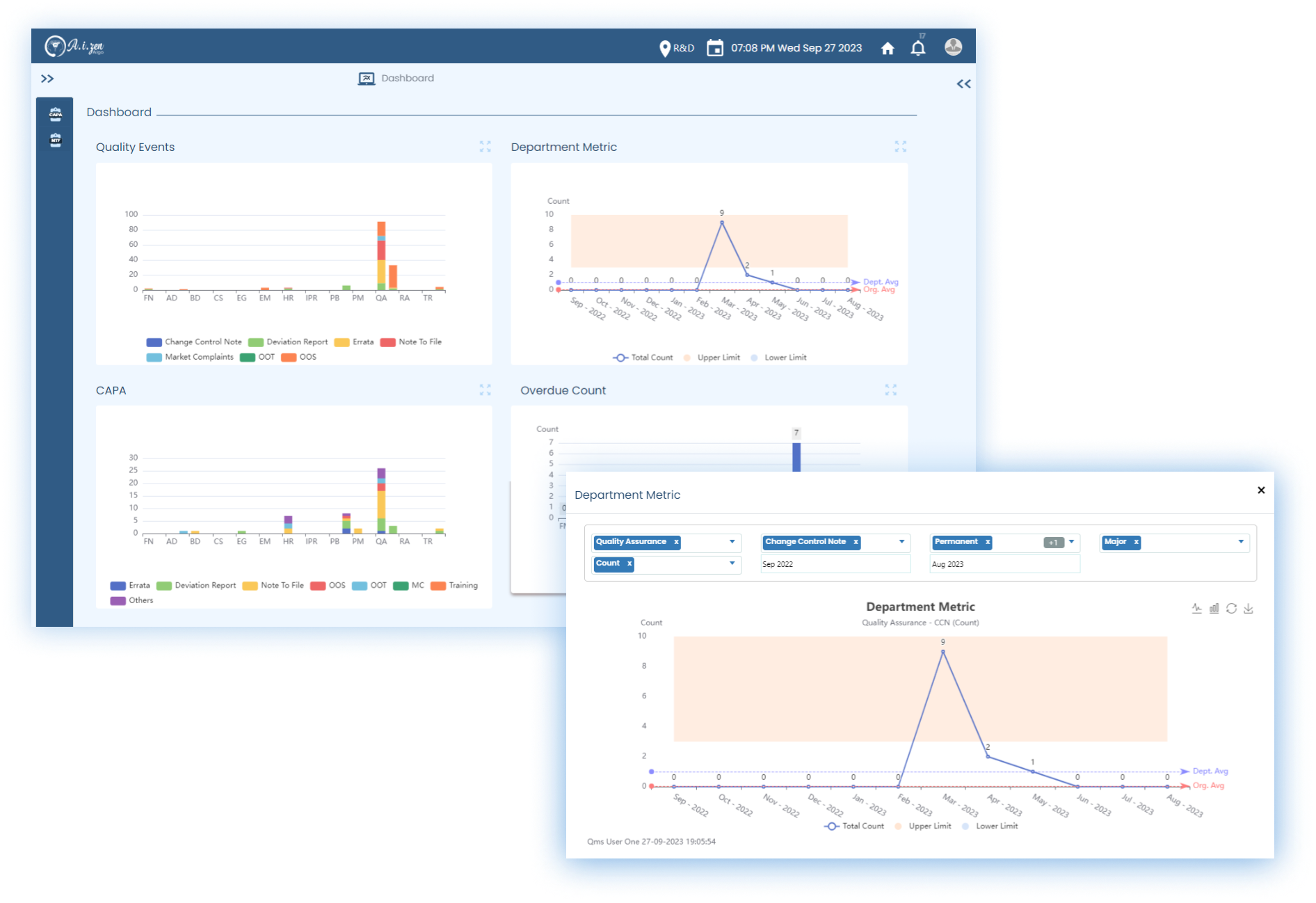
DocuZen TM
State of the art DMS that digitizes the documents - Creation &
Conversion, and automates the Business Workflows, Versioning, Storage, Retrieval, and
Distribution.
- 21 CFR Part 11 Compliant
- AI / ML enabled Workflows
- Efficiency through Automation
- Regulatory Compliance and Cost Savings

QualZenTM
GMP Compliant, Risk Mitigating - Quality Management System that
automates quality processes and procedures across departments of an enterprise.
- Compliance Risk Mitigation
- Cross Functional / Departmental Coordination
- Incident Management and Trends
- Quality Management across Product Lifecycle

VendorZenTM
Vendor Management System platform eases the Vendor Audits &
Evaluation
Processes for a risk-free procurement for an organization.
- Initial Assessments
- Scheduling
- Audits (Onsite/Remote)
- Audit Report Generation
- Periodic Evaluations
- Vendor Ratings

ClinZenTM
A Platform which integrates the Clinical Study Protocol Creation to
the Volunteer Management to the Laboratory Label Management for seamless clinical study
execution.
- Protocol Automation
- Volunteer Tracking
- Barcodes for chain of custody tracking

NoteZenTM
Streamline Your Research Workflow with Our Advanced Electronic Lab
Notebook.
Welcome to our state-of-the-art Electronic Lab Notebook (ELN)
platform, designed to
revolutionize the way you document, organize, and manage your scientific experiments and
research data. Our intuitive and customizable ELN solution is tailored to meet the unique
needs
of research laboratories across various disciplines.
- Creation of projects
- Creation of Experiments under the project
- Auto generation of experiment number
- Workspace for experiment creation

AuditZenTM
Manages entire audit lifecycle for all kinds of audits, including
planning and scheduling. We can Manage following type of audits in AuditZenTM
- Intuitive workflow for approval of audit reports and observations.
- Assigning observations to cross functional teams for their response.
- Department specific observations reports can be generated.
- Before closing the audit, a detailed report with observations and responses can able to
generate and export.
- AuditZen enables the industry to store all data inputs of audit cycle
- And facilitate the industries to rectify the already raised observations for the future.
- With ML model when observation is added into system it flashes the related previous
observations.
- Compliance with 21 CFR PART 11

EduZenTM
Easy-to-implement software for handling internal and external
training
requirement of the organization.
The system enables:
- Scheduling, tracking and reporting of employee training for the individual or workgroups.
- Complete, real-time visibility into the employees’ performance.
- Automation, increased efficiency, significant cost savings, enhanced security and improved
regulatory compliance.
- AI enabled selective training for employees’ skills enhancement

LabZenTM
A comprehensive end-to-end automated Laboratory Information
Management
System (LIMS) with Standalone, ZenVectorTM and Other Industry Standard Interfaces
Enterprise-wide
Systems.
- Chemical & Microbiology Analysis Lifecycle
- Investigative Analysis Lifecycle
- Comprehensive Resource Management
- Wide Variety of Equipment Interfaces
- Stability Studies

XeZenTM
An orchestration platform that transforms paper-based manual
processes
into digitized automated ones with Identification, Authentication, Verification and
Authorization. A versatile module that can be deployed - Standalone, Pluggable or Across
Platform.
- Digitization and Data Capture
- Multi Level / Multi Cycle Workflows
- Tasks Scheduling, Monitoring & Management
- Operational & Business Data Analytics